Colleges
- American Athletic
- Atlantic Coast
- Big 12
- Big East
- Big Ten
- Colonial
- Conference USA
- Independents (FBS)
- Junior College
- Mountain West
- Northeast
- Pac-12
- Patriot League
- Pioneer League
- Southeastern
- Sun Belt
- Army
- Charlotte
- East Carolina
- Florida Atlantic
- Memphis
- Navy
- North Texas
- Rice
- South Florida
- Temple
- Tulane
- Tulsa
- UAB
- UTSA
- Boston College
- California
- Clemson
- Duke
- Florida State
- Georgia Tech
- Louisville
- Miami (FL)
- North Carolina
- North Carolina State
- Pittsburgh
- Southern Methodist
- Stanford
- Syracuse
- Virginia
- Virginia Tech
- Wake Forest
- Arizona
- Arizona State
- Baylor
- Brigham Young
- Cincinnati
- Colorado
- Houston
- Iowa State
- Kansas
- Kansas State
- Oklahoma State
- TCU
- Texas Tech
- UCF
- Utah
- West Virginia
- Illinois
- Indiana
- Iowa
- Maryland
- Michigan
- Michigan State
- Minnesota
- Nebraska
- Northwestern
- Ohio State
- Oregon
- Penn State
- Purdue
- Rutgers
- UCLA
- USC
- Washington
- Wisconsin
High Schools
- Illinois HS Sports
- Indiana HS Sports
- Iowa HS Sports
- Kansas HS Sports
- Michigan HS Sports
- Minnesota HS Sports
- Missouri HS Sports
- Nebraska HS Sports
- Oklahoma HS Sports
- Texas HS Hoops
- Texas HS Sports
- Wisconsin HS Sports
- Cincinnati HS Sports
- Delaware
- Maryland HS Sports
- New Jersey HS Hoops
- New Jersey HS Sports
- NYC HS Hoops
- Ohio HS Sports
- Pennsylvania HS Sports
- Virginia HS Sports
- West Virginia HS Sports
ADVERTISEMENT
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Place to put my Nonsense Thread.
- Thread starter lurkeraspect84
- Start date
Whenever I hear the "Napa know how" jingle, in my head, I always sing "nappy dugout."
FDA Approves First Artificial Tumor

WASHINGTON—Following years of research and testing, the U.S. Food and Drug Administration officially approved a groundbreaking artificial tumor Tuesday, marking the first time a synthetic malignant growth has been cleared for use in patients across the country. “There were obviously significant complications in devising a tumor substitute the human body would accept, but we now have an artificial neoplasm that serves the same physiological functions as an organic abnormal growth of tissue,” said Jeffrey Shuren of the FDA’s Center for Devices and Radiological Health, adding that the polymer-based lump can be safely implanted in patients regardless of age, health, or medical history during a minimally invasive and relatively quick two-hour surgical procedure. “This synthetic tumor is remarkably lightweight, malignant, and capable of naturally metastasizing throughout the body. It also has the benefit of being incredibly small—roughly the size of a dime—but once in the body, it will grow two to three times in size and will get to work immediately replicating itself.” Shuren added that while initial prototypes of the device had only enough battery power to last a few days, the approved version is capable of going for several years or more, or until its objective has been completed.

WASHINGTON—Following years of research and testing, the U.S. Food and Drug Administration officially approved a groundbreaking artificial tumor Tuesday, marking the first time a synthetic malignant growth has been cleared for use in patients across the country. “There were obviously significant complications in devising a tumor substitute the human body would accept, but we now have an artificial neoplasm that serves the same physiological functions as an organic abnormal growth of tissue,” said Jeffrey Shuren of the FDA’s Center for Devices and Radiological Health, adding that the polymer-based lump can be safely implanted in patients regardless of age, health, or medical history during a minimally invasive and relatively quick two-hour surgical procedure. “This synthetic tumor is remarkably lightweight, malignant, and capable of naturally metastasizing throughout the body. It also has the benefit of being incredibly small—roughly the size of a dime—but once in the body, it will grow two to three times in size and will get to work immediately replicating itself.” Shuren added that while initial prototypes of the device had only enough battery power to last a few days, the approved version is capable of going for several years or more, or until its objective has been completed.
FDA Approves First Artificial Tumor

WASHINGTON—Following years of research and testing, the U.S. Food and Drug Administration officially approved a groundbreaking artificial tumor Tuesday, marking the first time a synthetic malignant growth has been cleared for use in patients across the country. “There were obviously significant complications in devising a tumor substitute the human body would accept, but we now have an artificial neoplasm that serves the same physiological functions as an organic abnormal growth of tissue,” said Jeffrey Shuren of the FDA’s Center for Devices and Radiological Health, adding that the polymer-based lump can be safely implanted in patients regardless of age, health, or medical history during a minimally invasive and relatively quick two-hour surgical procedure. “This synthetic tumor is remarkably lightweight, malignant, and capable of naturally metastasizing throughout the body. It also has the benefit of being incredibly small—roughly the size of a dime—but once in the body, it will grow two to three times in size and will get to work immediately replicating itself.” Shuren added that while initial prototypes of the device had only enough battery power to last a few days, the approved version is capable of going for several years or more, or until its objective has been completed.
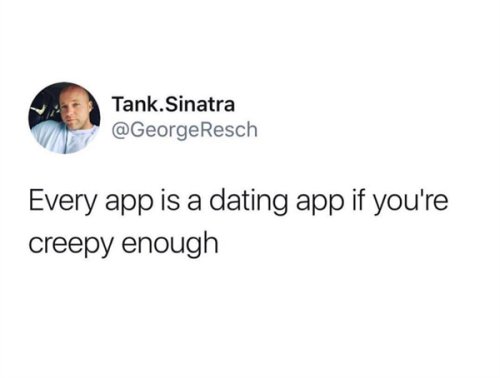
"For the guy who has everything."
Took the nail clippers with it apparently. Hot damn.
Anyone want to spray Ozzy with water, and feed him so he duplicates?
Similar threads
- Replies
- 15
- Views
- 801
- Replies
- 781
- Views
- 28K
- Replies
- 30
- Views
- 3K
- Replies
- 10
- Views
- 575
ADVERTISEMENT